May 8, 2024
Psychedelic Research for Advanced Cancer
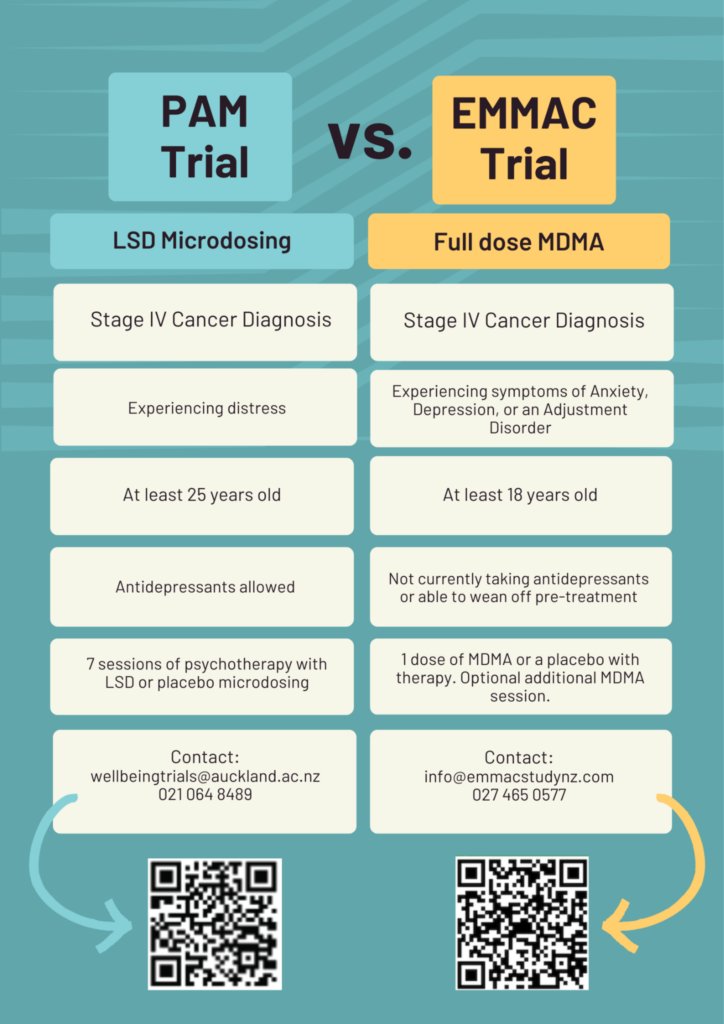
The University of Auckland and Otago got in touch with us recently about a research opportunity that offers support to people with an advanced cancer diagnosis. Both studies both involve receiving psychotherapy alongside a psychedelic. You can find out more about being part of this research below.
Some more detail about the trials:
PAM trial: This study compares the combination of a talk therapy called meaning centred psychotherapy alongside repeated very small doses of LSD versus the meaning centred psychotherapy combined with a placebo. The aim of this trial is to look at the effects of this intervention on psychological wellbeing and cancer-related distress. Research has already shown this talk therapy to be beneficial to people with cancer, so although half of our participants will be receiving the placebo and not the active LSD, we hope that all participants will get some benefit from their involvement in the trial. Sessions take place weekly for 7 weeks at our Auckland clinic.
EMMAC trial: This study compares the combination of psychotherapy alongside the drug MDMA versus psychotherapy combined with a placebo drug. The aim of this study is to examine the effects of MDMA-assisted therapy on the well-being and peace of mind of participants with Stage 4 cancer. A team of specialist clinicians will be treating 32 selected participants with late-stage cancer and monitoring the results of treatment. All participants have the opportunity to take part in an open label extension which means that whether you’ve received MDMA or the placebo, everyone is offered a treatment one month later with MDMA. Of course, there is no obligation to do this second treatment. Sessions take place at either our Auckland or Dunedin clinics.
Your participation in either trial will help in developing a new treatment to improve the mental wellbeing of people with cancer. If you have any questions or would like to know more, please get in touch with us using the information below.
PAM
Study Coordinator: Alesha Wells
Email: wellbeingtrials@auckland.ac.nz
Phone: 021 064 8489
Study Website: aleshawells6.wixsite.com/website
EMMAC
Study Coordinator: Will Evans
Email: info@emmacstudynz.com
Phone: 021 848 788
Study Website: www.emmacstudynz.com




